[PDF] Generic And Innovator Drugs A Guide To FDA Approval Requirements Ebook
Generic And Innovator Drugs A Guide To Fda Approval Requirements Aspen Publishers

Download Generic And Innovator Drugs A Guide To Fda Approval Require
Generic and Innovator Drugs: A Guide to FDA Approval ... Generic and Innovator Drugs: A Guide to FDA Approval Requirements, Eighth Edition provides step-by-step guidance of the approval process and expert interpretation of: The Hatch-Waxman Act (Drug Price Competition and Patent Restoration Act) The Medicare Prescription Drug, Improvement, and Modernization Act The Food and Drug Administration Modernization Act The FDA Export Reform and Enhancement Act The Biologics Price Competition and Innovation Act And more! Generic and Innovator Drugs: A Guide to FDA Approval ... Generic and Innovator Drugs: A Guide to FDA Approval Requirements, Eighth Edition provides step-by-step guidance of the approval process and expert interpretation of: The Hatch-Waxman Act (Drug Price Competition and Patent Restoration Act) The Medicare Prescription Drug, Improvement, and Modernization Act Generic and Innovator Drugs: A Guide to FDA Approval ... Generic and Innovator Drugs: A Guide to FDA Approval Requirements, Eighth Edition provides step-by-step guidance of the approval process and expert interpretation of: The Hatch-Waxman Act (Drug Price Competition and Patent Restoration Act)

Generic And Innovator Drugs A Guide To Fda Approval Requirements Eighth Edition Wolters

Anda Filing

Abbreviated New Drug Application Anda
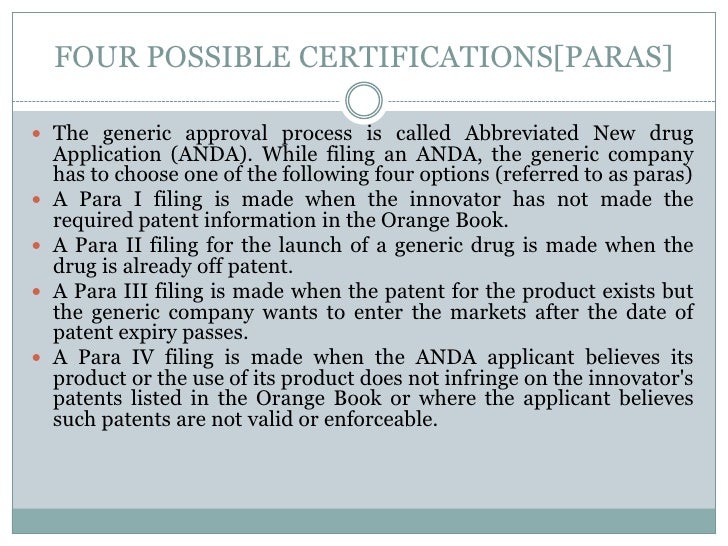
Para I Iv Orange Book
Overview Of Mental Health Medications For Children And Adolescents Ppt Video Online Download

0 Response to "Generic And Innovator Drugs A Guide To FDA Approval Requirements"
Post a Comment